How Phosphorous Affects Your Lawn and Environment
- "The Lawn Care Nut"July 15, 2023
You all who follow my teachings and strategies know that the two elements in fertilizer that I talk about most often are nitrogen and iron. That’s because these are the two that are going to give your lawn the deepest, darkest blue-green color response. And that is one reason why I’ve always liked using Milorganite because it contains 2.5% chelated iron along with 6% slow-release nitrogen to not only green up the grass but help the turf pull in that iron to green up even more.
But there is another element in Milorganite that is equally important. In fact, it’s a macronutrient so one could argue that in bulk, it’s needed much more than iron. That element is phosphorus.
It’s the second number on the bag of Milorganite which is four. That 4 stands for 4% and lets you know that in every bag of Milorganite, 4% of it is phosphorus.

What Does Phosphorus Do For Grass Plants?
When I was taught about fertilizers for lawns and the big 3 macronutrients (the 3 numbers on the bag) I learned “Up” “Down” and “All Around” as a way to know what each of these does for plants. Nitrogen turns plants green and gives them top growth, phosphorus builds strong roots and potassium helps with all-around health and balance.
That’s a pretty good way to describe it, for sure, but it’s pretty elementary.
I wanted to see if there was a better way to explain it, so I asked ChatGPT to explain it to me as if I were a high school student. Here’s what I got:
Explain to me what phosphorus does for plants and explain it to me at a high school level.
Certainly! Phosphorus plays a vital role in the growth and development of plants at a cellular level. Let's dive into it at a high school level.
Phosphorus is an essential nutrient for plants, meaning they need it to survive and thrive. It is a component of many important molecules in plants, such as DNA, RNA, and ATP (adenosine triphosphate), which is the energy currency of cells. This nutrient is involved in various physiological and metabolic processes within plants.
One of the primary functions of phosphorus is its role in energy transfer and storage. ATP, as I mentioned earlier, is responsible for storing and providing energy for plant cells. When sunlight is absorbed during photosynthesis, phosphorus-containing molecules help convert that energy into chemical energy stored in ATP. This energy is then utilized by the plant to carry out essential processes like growth, reproduction, and nutrient transport.
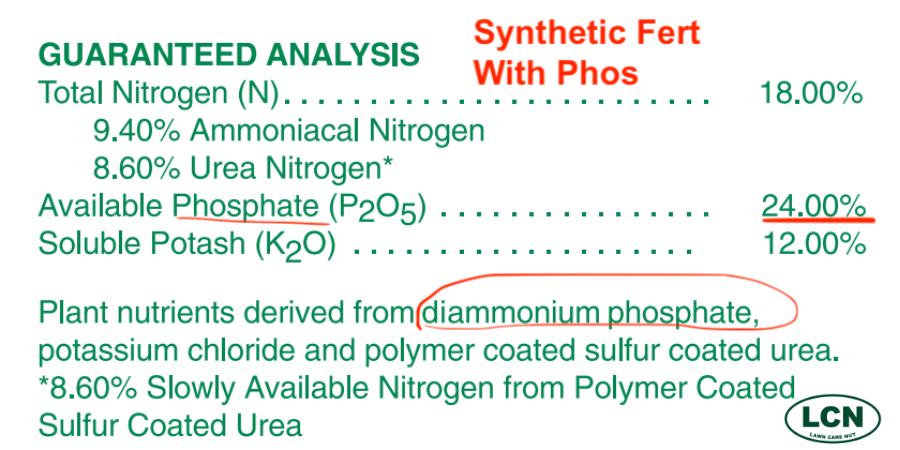
This is the guaranteed analysis from a common synthetically derived and blended fertilizer containing phosphorus.
Additionally, phosphorus is crucial for root development and function. It promotes root growth by aiding in the formation of new cells and supporting the elongation of root hairs. These root hairs are tiny projections that increase the surface area of roots, allowing plants to absorb water and nutrients more efficiently from the soil. Phosphorus also helps in the synthesis of certain compounds that regulate root architecture and enable the plant to explore and extract nutrients from the soil effectively.
Furthermore, phosphorus is involved in the transfer and storage of genetic information. It plays a fundamental role in DNA and RNA, the molecules responsible for carrying and expressing genetic information in cells. Phosphorus is a key component of the backbone of these molecules, providing structural stability and allowing for the accurate transmission of genetic instructions during cell division and growth.
In summary, phosphorus is a vital nutrient for plants, fulfilling multiple critical functions. It contributes to energy production, supports root growth and nutrient uptake, and participates in genetic processes necessary for plant growth, development, and reproduction.
For the record, that is my first time ever personally using the OpenAI Chatbot and I am thoroughly impressed.
Where Does Phosphorus Come From?
There are a few different sources of phosphorus on this planet. The one that I first think of is called “bone meal” and my Grandma swore by it in her garden. Every new planting got a dusting of bone meal in the hole before placing the plant. It comes from exactly what it states, “crushed bones.”
Another popular source of phosphorus is bird guano (poop). Not all guano is created equally as early fertilizer prospectors learned when they claimed nearly 200 remote islands for the harvesting of bird guano for fertilizer. Here is an interesting article from the Smithsonian.
There is also some phosphorus in cow and chicken manure which is used mostly in organic farming, as well as composting.
Rock Phosphate Mining
Today, most of the phosphorus used in fertilizers for crops and lawns comes from rock phosphate that is mined in Florida. In fact, 75% of the phosphate used in the United States, as well as about 25% of the phosphate used around the world (source) is mined here in Florida, about 15 miles from where I live in Manatee County.
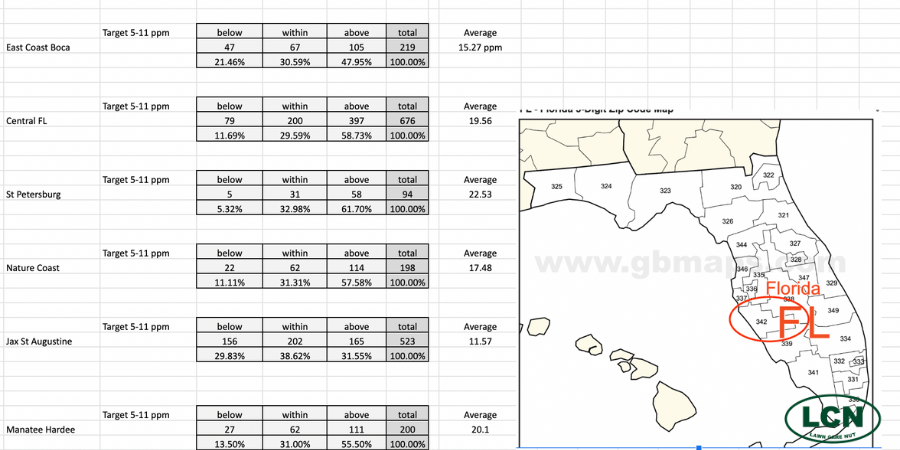
Here you can see soil test data showing phosphorus levels across the state of Florida. Note the panhandle where phosphorus levels are very low compared to the rest of the state.
In order for plants to utilize the phosphorus mined, the rock phosphate must go through a few different processes from washing to separating (beneficiation) to reacting it with sulfuric acid to produce phosphoric acid.
The most common fertilizer product used in lawn fertilizers is diammonium phosphate (DAP), made by reacting ammonia with phosphoric acid. This yields a source of phosphorus that is water soluble and available for plant uptake right away.
There can be a downside here though, and that being that the phos is so readily available, it can be easily washed out of soil or leached through. In Florida for example, we get heavy afternoon rainstorms during summer and these can wash phosphorus off target, into streets, to storm drains, and out into the rivers and the gulf or Atlantic feeding algal blooms and worsening red tide. This is such a concern here that many counties have enacted fertilizer ordinances that restrict the application of nitrogen and phosphorus fertilizers through our rainy season June - September.
Phosphorus in Milorganite
The phosphorus in Milorganite is plant available but since it’s from a natural source, it’s slow release. The phosphorus is literally bound into a homogenous prill that will only release nutrients slowly as heat, friction, and microbes break them down. This is one reason why you don’t have to “water in” your Milorganite after you apply it. The prills are not water-soluble. Instead, they are slow release which reduces the chances for leaching. You can read more about the P in Milorganite here, including a study conducted by the University of Florida in 2007.
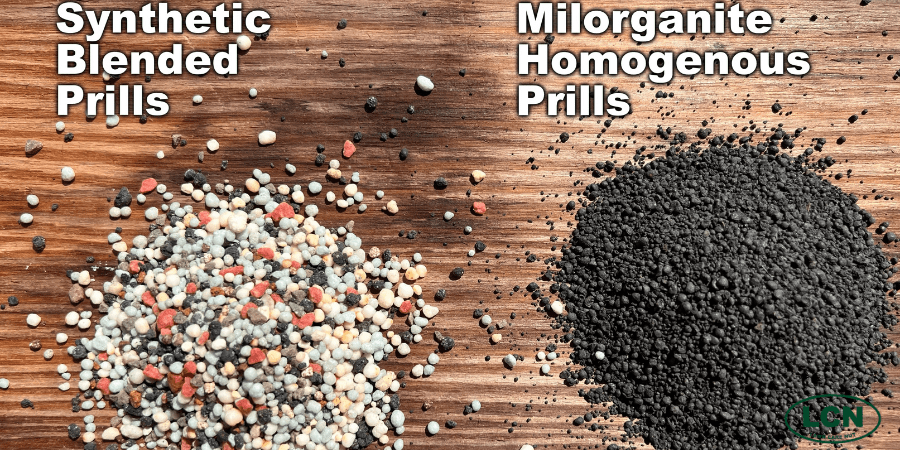
Side-by-side of a synthetically derived fertilizer with blended nutrients vs. the homogenous prills of Milorganite. With Milorganite, each prill contains 100% of the analysis and it’s all slow release.
How You Can Help Avoid Runoff into Waterways When Fertilizing:
- Avoid fertilizing within 10-25 feet of any waterways.
- Avoid fertilizing if heavy rain is in the forecast.
- Never dispose of fertilizer in waterways, wetlands, or other bodies of water.
- After fertilizing, sweep excess fertilizer from solid surfaces, such as driveways, decks, patios, streets, and sidewalks, back onto the lawn.
- Create a buffer zone of grasses and natural vegetation along the shoreline to help prevent soil erosion and retain nutrients that might otherwise enter a waterway.
How To Know If You Need Phosphorus
The best way to know if phosphorus will be beneficial to your lawn strategy is to conduct a soil test. Since phosphorus is mined here, I decided to look at soil test data from all around Florida to see what was revealed. And while I found that our averages in most areas were very high; 20% of the samples taken still showed low phosphorus.
Additionally, in the panhandle of Florida (Northwest Florida) the naturally existing phosphorus is quite low across most of the samples. This has to do with how Florida was formed and the study of “plate tectonics.” Lots of interesting reading here.
At the end of the day, plants need phosphorus. It’s one of the big 3 macronutrients and no matter who or what explains it, we know it’s all about those roots. The thicker the roots, the thicker the top and if you are going to apply phosphorus to help with this, Milorganite is and always has been a great choice.
I’ll see you in the lawn!

